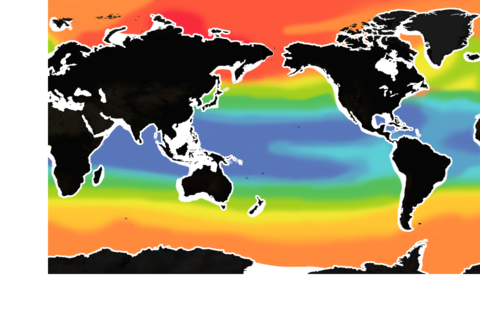
Our oceans and coastal waters are under extreme stress, emanating from multiple sources:
Global Warming
Deoxygenation
Rising Sea Level
Plastic Leakage
Chemical Pollution
De-forestation
As the ocean absorbs ever more carbon dioxide from the atmosphere, the pH level in many of its seawaters is falling. In other words, their acidity is increasing.
Ocean acidification poses an existential threat to many forms of marine life, and thus to food chains, livelihoods and economies. What is it, and what can we do to avoid its worst impacts?
Global Warming
Deoxygenation
Rising Sea Level
Plastic Leakage
Chemical Pollution
De-forestation
Less familiar than most of these, but potentially as or more catastrophic to some marine life, is acidification.
With the exception of 2020, emission volumes have risen
consistently over time.
bn tonnes
2021
2020
2000
1950
1900
1850
1800
1750
Data from: Andrew, Robbie M., & Peters, Glen P. (2022). The Global Carbon Project's fossil
CO2 emissions dataset (2022v27). Zenodo.
Carbon forms a natural part of many biological processes. Its absorption from the atmosphere, and its sequestering in these processes over long time-scales, mitigates the extent of global warming.
But the rate at which we are emitting CO2 into the atmosphere is rising dramatically, and the oceans can’t sequester it quickly enough. The result is an increase in ocean acidity.
If acidification continues to worsen at its current rate, the malign impact on humanity – especially our food chains and our economies – will be significant.
“
Director of Science, Plymouth Marine Laboratory
Co-Chair, Global Ocean Acidification Observing Network (GOA-ON)
Co-ordinator, Ocean Acidification Research for Sustainability (OARS) programme
How acidification impacts ocean chemistry
When reaching the ocean surface, CO2 dissolves and reacts with water molecules to form carbonic acid (H2CO3).
… depletes the carbonate that some marine organisms need to form the hard outer structures which protect them from predators
… can reduce organisms’ fitness, altering their growth, reproduction and life span
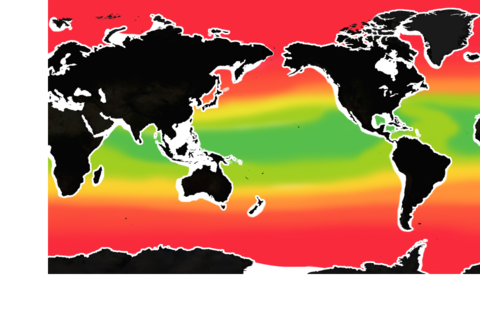
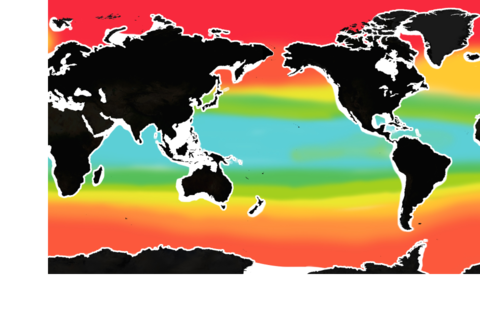
The pH level of seawater is one of the key indicators of ocean acidification. (The pH scale runs from 0 to 14.)
The mean surface ocean pH level has fallen from 8.2 to below 8.1. About half of that decline has occurred in the past 40 years – a sign that acidification has been accelerating.
Data and timescale image from: ETH Zurich
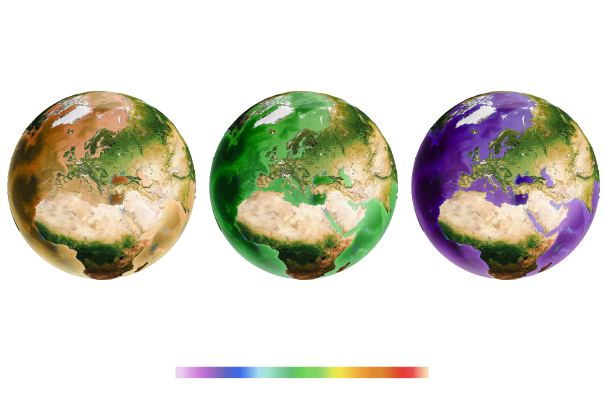
But pH is already lower than 8 in some waters, especially along some of our coasts.
If global carbon emissions continue to increase at their current rate, by 2100, pH will be below 8 in most of the ocean.
Courtesy of Andrew Yool. Adapted from HS Findlay and C Turley 2021. Ocean acidification and climate change. In: TM Letcher (Ed), Climate change: observed impacts on Planet Earth (Third Edition)
Measuring acidification is more complex along coastlines than in the open ocean, where pH levels do not vary widely. Variation is considerably greater in coastal waters.
There are three main reasons:
In some areas, such as along the Pacific coasts of North and South America and the coast of Northwest Africa, deep CO2-rich water periodically rises toward the surface and toward the shore.
Photosynthesis, for example, consumes CO2 from seawater. And decomposition of organic matter releases more CO2 into the water.
Sewage discharge and chemical run-off, for example, can combine with CO2 absorption to increase surface water acidification.
“
Senior Research Scientist
Pacific Marine Environmental Laboratory
National Oceanic and Atmospheric Administration (NOAA)
Aragonite is a naturally occurring form of calcium carbonate (CaCO3) that molluscs use to form their shells and corals their endoskeletons.
The less saturated a body of water is with aragonite, the more corrosive it is likely to be. And the more energy such organisms must expend to develop the outer structures they need to survive.
When the aragonite saturation state falls below a value of 3, these organisms become stressed. When it falls below 1, unprotected shells and other aragonite structures begin to dissolve.
Saturation state:
3.2725
1982
Data from: OceanSODA, ETHZ (ETH Zurich)
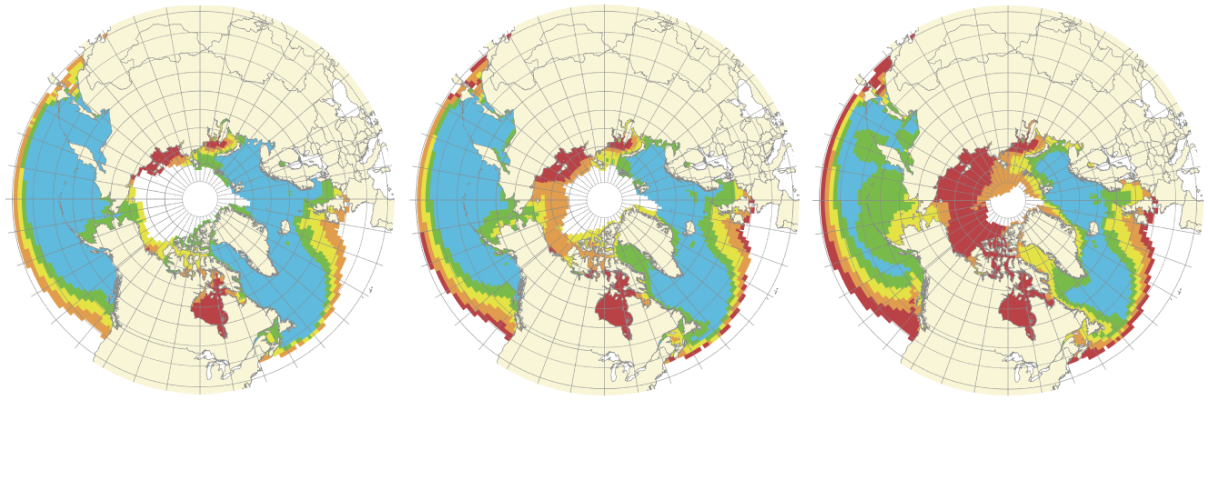
Under-saturation is already a serious threat to organisms in the Arctic and Southern Oceans, where the saturation state is close to 1. (Colder waters absorb CO2 more easily than do warmer waters.)
(Note: future projections are based on the assumption of unmitigated emissions growth from existing levels)
2000
Adapted from R Danovaro et al, The Significance and Management of Natural Carbon Stores in the Open Ocean (2014)
“
We cannot today predict with any precision the extent of damage ocean acidification will cause in the future. However, the biological and economic impacts of ocean acidification are already documented in some coastal areas. Such cases provide a hint of the damage to marine ecosystems and economies that could await if we fail to take action – above all, to arrest and reverse the growth of CO2 emissions.
Parts of the oyster aquaculture industry in the Pacific Northwest have come under direct threat from acidification over the past 15 years.
Oyster hatcheries in Whiskey Creek, Oregon and Dabob Bay, Washington became unable to produce oyster larvae. The reason: the larvae died due to the acidification of the inflowing water.
Scientists helped the hatcheries to decrease the acidity of their inflowing seawater. This helped to save the hatcheries and the jobs they provide.
Shellfish farming in the Pacific Northwest (in 2015)
Economic activity
Jobs
Economic activity and job figures from: A Barton, G Waldbusser, R Feely et al, Impacts of Coastal Acidification on the Pacific Northwest Shellfish Industry and Adaptation Strategies Implemented in Response (2015)
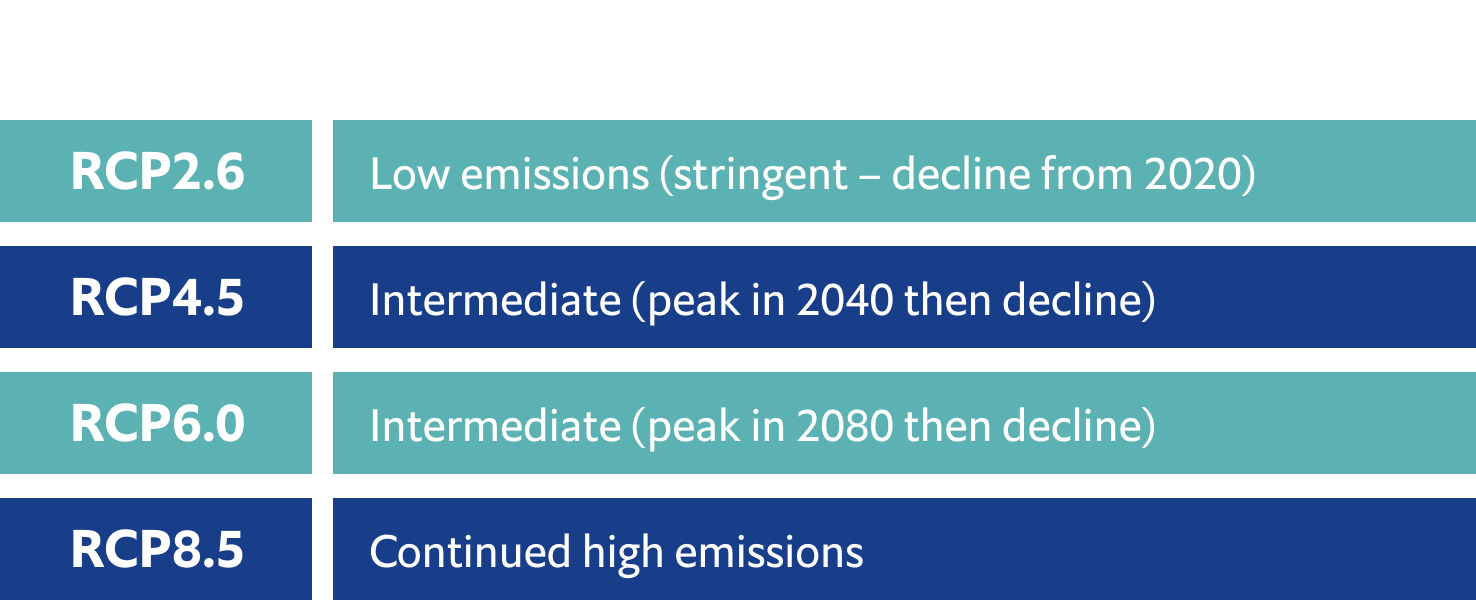
Scientists are modelling the possible future impacts of acidification. Their projections are based on the four main climate change scenarios (RCPs – Representative Concentration Pathways) developed by the UN Intergovernmental Panel on Climate Change (IPCC).
Even under a low-emissions scenario, warm-water corals and some polar species will be at high risk of impact by 2100. But if if emissions remain high, many additional organisms and habitats will be at very high risk by then, and some by 2050.
Text and illustration adapted from JF Gattuso et al, “Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios”, Science 349 (2015)
Also known as “sea butterflies”, they play a vital role in marine ecosystems and food chains.
At higher latitudes, pteropods form a vital part of the diet of commercially important fish such as salmon.
Modelling suggests that, under the high-emissions scenario (RCP8.5), high-latitude pteropods (Lima helicina) will be at very high risk by 2050.
Adapted from S Comeau, R van Hooidonk in onesharedocean.org
The economic impacts of ocean acidification are yet to be widely studied. But some experts have modelled potential country or regional losses from the decline of shellfish production by 2100 based on different high-emissions climate-change scenarios.
£23m - £88m
United Kingdom
S Mangi, et al, The Economic Impacts of Acidification on Shellfish Fisheries and Aquaculture in the United Kingdom (2018)
$75m - $187m
United Kingdom
S Cooley & S Doney, Anticipating ocean acidification’s economic consequences for commercial fisheries (2009)
~$400m
United States
D Narita, K Rehdanz, R Tol, Economic costs of ocean acidification: a look into the impacts on global shellfish production (2012)
>$1bn
Europe
D Narita & K Rehdanz, Economic impact of ocean acidification on shellfish production in Europe (2016)
“
Deputy Director
Laboratoire d’Océanographie de Villefranche (LOV)
Fighting ocean acidification is integral to one of the UN Sustainable Development Goals, SDG 14, which aims to conserve and sustainably use the oceans, seas and marine resources for sustainable development.
SDG target 14.3: “Minimize and address the impacts of ocean acidification, including through enhanced scientific cooperation at all levels”
We can turn back the tide of acidification, but we must act quickly.
We must:
Reduce carbon emissions
Build ecosystem resilience
Step up research efforts
This is priority one. If we continue emitting CO2 into the atmosphere at current or higher levels, resilience-building and research efforts will help only little.
To reach Net Zero emissions by mid-century, 2010 levels of global CO2 emissions must fall by 45% by 2030.
If we can reduce atmospheric CO2 emissions dramatically, we can avoid the most severe impacts of ocean acidification. This outcome is within our control. If we respond quickly and effectively at the local, national and international levels, we can achieve it, but time is short. We must act now.
Marine protected areas (MPAs) – set aside by governments for long-term conservation – can be made “climate smart” by using them to develop methods to mitigate or adapt to the effects of ocean warming and acidification. Climate-smart MPAs should, for example, host efforts to further develop carbon-sequestration methods.
The UK, among other countries, aims to expand the use of “climate-smart” decision-making for its MPAs.
Pilots are also under way to develop methods of extracting carbon directly from seawater, including through the use of renewable energy. If successful, such projects will need to be scaled up to make a real contribution to reducing ocean carbon.
Nature-based solutions such as blue carbon habitats can help slow acidification. In coastal wetlands, for example, mangroves draw carbon from the atmosphere, reducing the amount reaching the water. And seagrasses take up carbon from seawater and store it away for long periods of time.
Large gaps remain in scientists’ understanding of ocean acidification and its impacts.
More monitoring and research are needed to, for example, better understand the conditions and impacts of acidification in coastal areas:
The extreme variability of carbonate chemistry along coastlines and how it interacts with different marine organisms
The interactions between these and the human communities living in proximity to the coast
More work is also needed to monitor, verify and evaluate the impact of carbon-removal and nature-based solutions.
“
for sharing with us their knowledge about ocean acidification and for their generous assistance in finding and accessing relevant data:
Director of Science, Plymouth Marine Laboratory
Co-Chair, Global Ocean Acidification Observing Network (GOA-ON)
Co-ordinator, Ocean Acidification Research for Sustainability (OARS) programme
Biological Oceanographer
Plymouth Marine Laboratory
Senior Research Scientist
Pacific Marine Environmental Laboratory
National Oceanic and Atmospheric Administration (NOAA)
Deputy Director
Laboratoire d’Océanographie de Villefranche (LOV)
Postdoctoral Researcher
Environmental Physics Group
ETH Zürich
A podcast series, led by Charles Goddard and Naka Kondo at Economist Impact, talks to leading experts around the world about the science of chemical pollution in the oceans - and the most promising pathways to tackle the scourge.
With less than ten years to achieve the 2030 Sustainable Development Goals, there is a renewed urgency to examine global systems and balance human aspirations with the planet’s ability to sustain them. This is why Economist Impact has launched The Sustainability Project, a content platform and community hub combining insights, innovation and influence. Our aim is to convene and engage global stakeholders who have the power to bring real change.
Economist Impact’s World Ocean Initiative imagines an ocean in robust health, and with a vital economy. Year-round and at our flagship World Ocean Summit, we foster a global conversation on the greatest challenges facing the seas, inspiring bold thinking, new partnerships and the most effective action to build a sustainable ocean economy.
We welcome your feedback and comments.
If you have an editorial or media related request, a member of the media team will get back to you.
Back to Blue explores evidence-based approaches and solutions to the pressing issues faced by the ocean, to restoring ocean health and promoting sustainability. Sign up to our monthly Back to Blue newsletter to keep updated with the latest news, research and events from Back to Blue and Economist Impact.
The Economist Group is a global organisation and operates a strict privacy policy around the world.
Please see our privacy policy here.
Thank you for your interest in Back to Blue, please feel free to explore our content.
If you would like to co-design the Back to Blue roadmap or have feedback on content, events, editorial or media-related feedback, please fill out the form below. Thank you.
The Economist Group is a global organisation and operates a strict privacy policy around the world.
Please see our privacy policy here.